Vanilla Callogenesis with the Addition of Picloram and BAP under Dark and Light Conditions
DOI:
https://doi.org/10.15294/unnesjlifesci.v14.i1.22275Keywords:
BAP, Callus, Lighting, Picloram, VanillaAbstract
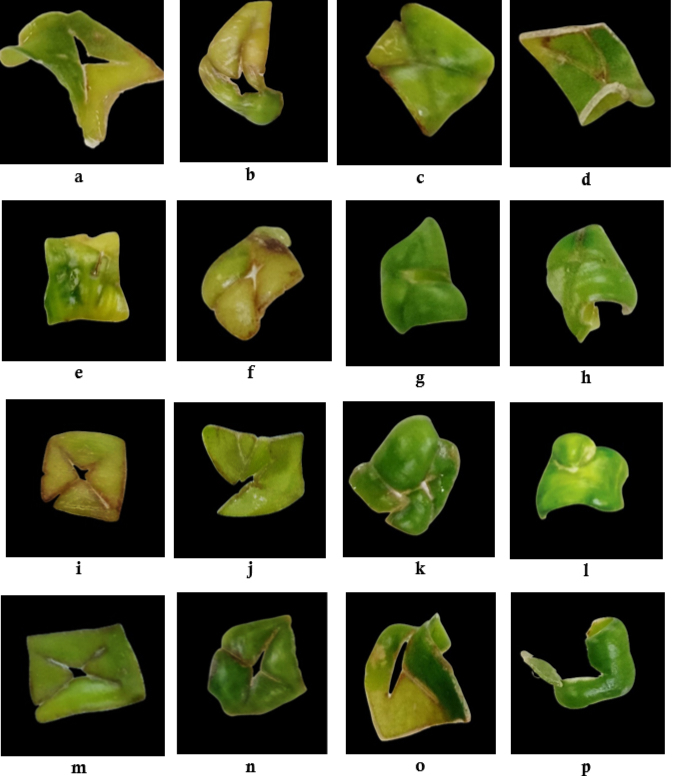
Vanillin is a secondary metabolite in vanilla, with a 1%–3% content. Tissue culture is an effective alternative for producing plant secondary metabolites. This study aimed to analyze the effect of picloram and BAP under light and dark conditions on the callogenesis of vanilla leaf explants. A randomized block design was used with two factors: picloram concentration (0 ppm, 2.5 ppm, 5 ppm, 7.5 ppm) and BAP (0 ppm, 1 ppm, 2 ppm, 3 ppm), as well as lighting conditions (light and dark). Vanilla leaf explants were cultured on MS medium and incubated for three months. The observed parameters included the time for explants to curve, the percentage of curved explants, the time of callus formation, the percentage of callus-forming explants, and callus morphology. The results showed that PGRs type/concentration significantly affected the percentage of curved explants but had no significant effect on other parameters. Lighting and its interaction with PGRs had no significant effect. The best combination of picloram and BAP resulted in 100% curved explants but was less optimal for inducing callus formation in vanilla leaf explants. This study can open scientific insights into the combination of picloram and BAP and the effects of different lighting in vanilla leaf callogenesis.
Downloads
References
Adil, M., Ren, X., & Jeong, B. R. (2019). Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. Journal of Photochemistry and Photobiology B: Biology, 196(May), 111509. https://doi.org/10.1016/j.jphotobiol.2019.05.006
Anjani, D. D., & Ratnawati. (2023). The Effect of BAP and NAA Combination on Callus Induction of Aglaonema Siam Aurora Leaf Explants in Vitro. Indonesian Journal of Bioscience (IJOBI), 1(2), 85–92. https://doi.org/10.21831/ijobi.v1i2.213
Chai, L. C., Alderson, P. G., & Chin, C. F. (2024). Exogenous Cytokinin Induces Callus and Protocorm-Like-Bodies (PLBs) Formation in In Vitro Root Tips of Vanilla planifolia Andrews. Tropical Life Sciences Research, 35(1), 233–256. https://doi.org/10.21315/tlsr2024.35.1.13
Deng, X., Xiong, Y., Li, J., Yang, D., Liu, J., Sun, H., Song, H., Wang, Y., Ma, J., Liu, Y., & Yang, M. (2020). The establishment of an efficient callus induction system for lotus (Nelumbo nucifera). Plants, 9(11), 1–13. https://doi.org/10.3390/plants9111436
Du, M., Spalding, E. P., & Gray, W. M. (2020). Rapid Auxin-Mediated Cell Expansion. Annual Review of Plant Biology, 71, 379–402. https://doi.org/10.1146/annurev-arplant-073019- 025907
Farhadi, N., Panahandeh, J., Azar, A. M., & Salte, S. A. (2017). Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of persian shallot (Allium hirtifolium). Scientia Horticulturae, 218, 80–86. https://doi.org/10.1016/j.scienta.2016.11.056
Fehér, A. (2019). Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Frontiers in Plant Science, 10(April), 1–11. https://doi.org/10.3389/fpls.2019.00536
Gnasekaran, P., Rahman, Z. A., Chew, B. L., Uddain, J., Solayappan, M., Chear, N. J. Y., Appalasamy, S., Mariappan, V., Wahyuni, D. K., & Subramaniam, S. (2023). Picloram enhanced the callus induction, growth kinetics, antioxidant potentials, and secondary metabolites production of Zingiber officinale var. rubrum callus cultures. Plant Cell, Tissue and Organ Culture, 155(3), 843–859. https://doi.org/10.1007/s11240-023-02603-6
Hardjo, P. H., Savitri, W. D., Artadana, I. B. M., Putra, S. E. D., Parac, E. P., & Jan, A. (2021). Callus-mediated Somatic Embryogenesis and Plant Regeneration in Vanda tricolor Lindl. var. Pallida. Jordan Journal of Biological Sciences, 14(5), 933–937. https://doi.org/10.54319/JJBS/140508
Hassan, M. M., Allam, M. A., Shams El Din, I. M., Malhat, M. H., & Taha, R. A. (2021). High-frequency direct somatic embryogenesis and plantlet regeneration from date palm immature inflorescences using picloram. Journal of Genetic Engineering and Biotechnology, 19(1), 33. https://doi.org/10.1186/s43141-021-00129-y
Khan, N., Ahmed, M., Hafiz, I., Abbasi, N., Ejaz, S., & Anjum, M. (2015). Optimizing the concentrations of plant growth regulators for in vitro shoot cultures, callus induction and shoot regeneration from calluses of grapes. Journal International Des Sciences de La Vigne et Du Vin, 49(1), 37–45. https://doi.org/10.20870/oeno-one.2015.49.1.95
Lawrence, P. K., Senarath, W. T. P. S. K., & Munasinghe, M. L. A. M. S. (2022). In vitro Callus Induction of Catunaregam spinosa Using Leaves as Explant. Journal of Pharmaceutical Research International, 34, 67–76. https://doi.org/10.9734/jpri/2022/v34i43a36313
Majda, M., & Robert, S. (2018). The role of auxin in cell wall expansion. International Journal of Molecular Sciences, 19(4). https://doi.org/10.3390/ijms19040951
Menon, S., & Nayeem, N. (2013). Vanilla planifolia : A Review of a Plant Commonly Used as Flavouring Agent. Int. Journal of Pharmaceutical Sciences Review and Research, 20(2), 225–228.
Mohammed, B., Bilooei, S. F., Dóczi, R., Grove, E., Railo, S., Palme, K., Ditengou, F. A., Bögre, L., & López-Juez, E. (2018). Converging light, energy and hormonal signaling control meristem activity, leaf initiation, and growth. Plant Physiology, 176(2), 1365–1381. https://doi.org/10.1104/pp.17.01730
Mohei, E.-D. S., Wael, F. S., Heba, A. A. M., Mohammed, I. A., Abdullatif, A. A.-K., Solliman, A. A.-K., Adel, E. A. H., & Hesham, M. A.-M. (2017). Induction of biochemical active constituents of Jojoba (Simmondsia chinensis (Link) Schneider) callus affected by hormones. Journal of Medicinal Plants Research, 11(2), 34–42. https://doi.org/10.5897/jmpr2015.6196
Murthy, H. N., Lee, E. J., & Paek, K. Y. (2014). Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell, Tissue and Organ Culture, 118(1), 1–16. https://doi.org/10.1007/s11240-014-0467-7
Oh, E., Zhu, J. Y., Bai, M. Y., Arenhart, R. A., Sun, Y., & Wang, Z. Y. (2014). Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. ELife, 2014(3), 1–19. https://doi.org/10.7554/eLife.03031
Ozel, C. A., Unal, F., Avuloglu-Yilmaz, E., Erikel, E., Mirici, S., & Yuzbasioglu, D. (2022). Determination of genotoxic damages of picloram and dicamba with comet assay in Allium cepa rooted in tissue culture and distilled water. Molecular Biology Reports, 49(12), 11273–11280. https://doi.org/10.1007/s11033-022-07712-7
Park, J., Lee, S., Park, G., Cho, H., Choi, D., Umeda, M., Choi, Y., Hwang, D., & Hwang, I. (2021). Cytokinin-responsive growth regulator regulates cell expansion and cytokinin-mediated cell cycle progression. Plant Physiology, 186(3), 1734–1746. https://doi.org/10.1093/PLPHYS/KIAB180
Pasternak, T. P., & Steinmacher, D. (2024). Plant Growth Regulation in Cell and Tissue Culture In Vitro. Plants, 13(2), 1–24. https://doi.org/10.3390/plants13020327
Phillips, G. C., & Garda, M. (2019). Plant tissue culture media and practices: an overview. In Vitro Cellular and Dev. Biology-Plant, 55(3), 242–257. https://doi.org/10.1007/s11627-019-09983-5
Rojas-López, A., & Cañizares-Macías, M. P. (2013). Antioxidant Capacity in Vanilla Extracts Obtained by Applying Focused Microwaves. Food and Nutrition Sciences, 04(08), 244–253. https://doi.org/10.4236/fns.2013.48a030
Sharma, R., & Bora, S. (2015). Comparative Studies on Callus Induction from Different Explants of Vanilla planifolia Andrews. Int. Journal of Advanced Biotechnology and Research, 6(3), 360–365.
Siddique, A. B., & Islam, S. S. (2018). Effect of light and dark on callus induction and regeneration in tobacco (Nicotiana tabacum L.). Bangladesh Journal of Botany, 44(4), 643–651. https://doi.org/10.3329/bjb.v44i4.38636
Silva, C. P. da, Peters, J. A., Pasa, M. da S., Danielowski, R., & Bianchi, V. J. (2017). In Vitro Regeneration of “Carrick” pear from Leaf Plants. UERGS, 3(1), 238–248. doi: http://dx.doi.org/10.21674/2448-0479.31.238-248
Suhartanto, B., Astutik, M., Umami, N., Suseno, N., & Haq, M. S. (2022). The effect of explants and light conditions on callus induction of srikandi putih maize (Zea mays L.). IOP Conference Series: Earth and Environmental Science, 1001(1), 1–5. https://doi.org/10.1088/1755-1315/1001/1/012006
Wahyudi, A., Sujianto, S., & Kurniasari, I. (2021). Strategy for developing Indonesian vanilla products to improve the added value. IOP Conference Series: Earth and Environmental Science, 892(1). https://doi.org/10.1088/1755-1315/892/1/012042
Wahyuni, D. K., Huda, A., Faizah, S., Purnobasuki, H., & Wardojo, B. P. E. (2020). Effects of light, sucrose concentration and repetitive subculture on callus growth and medically important production in Justicia gendarussa Burm.f. Biotechnology Reports, 27, e00473. https://doi.org/10.1016/j.btre.2020.e00473
Wit, M. de, Galvão, V. C., & Fankhauser, C. (2016). Light-Mediated Hormonal Regulation of Plant Growth and Development. Annual Review of Plant Biology, 67, 513–537. https://doi.org/10.1146/annurev-arplant-043015-112252