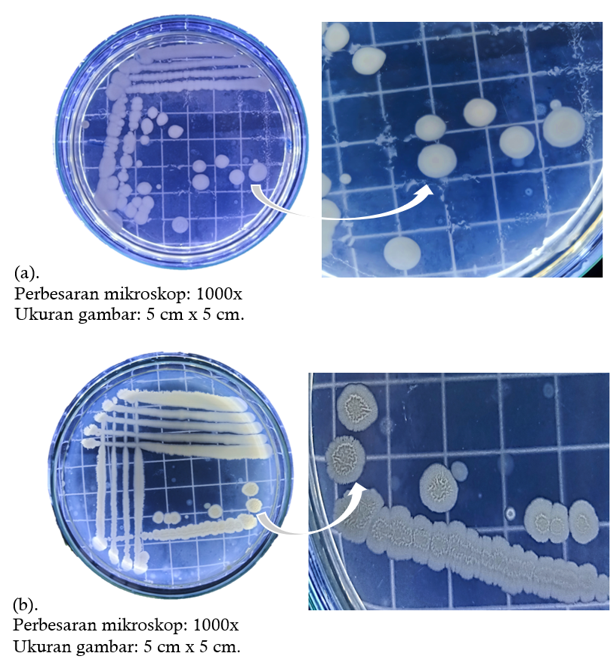
Pengaruh Variasi Metode Ekstraksi Metabolit Sekunder Bakteri Endofit Daun Rambutan (Nephelium lappaceum L.) terhadap Pertumbuhan Escherichia coli dan Bacillus subtilis
DOI:
https://doi.org/10.15294/unnesjlifesci.v13.i2.2327Keywords:
Antibacterial, Endophytic bacteria, Maceration, Methanol, Rambutan leafAbstract
Rambutan (Nephelium lappaceum L.) is one of the many tropical plants found in Indonesia. The leaves of the rambutan plant are known to be used as medicine, so they have the potential to be a source of endophytic bacteria that can produce beneficial compound. This study aims to isolate, identify of endophytic bacteria from rambutan leaves, and test their activity as an antibacterial with two different secondary metabolite extraction methods, namely maceration with 70% methanol and without maceration. This study used experimental research with a Completely Ramdomized Design (CRD). Isolation of endophytic bacteria from rambutan leaves produced two isolates (D1 and D2). The results of identification through macroscopic, microscopic, and biochemical test showed that two pure isolates had similarities to genus Bacillus sp. Antibacterial activity testing was carried out using well diffusion method. Based on the results of antibacterial test showed that extraction method with 70% methanol produced an extract that was more effective as an antibacterial with average diameter of clear zone formed for Escherichia coli bacteria were 13.55 mm (Isolate D1) and 12.13 mm (Isolate D2) and that of Bacillus subtilis bacteria were 16.27 mm (Isolate D1) and 19.69 mm (Isolate D2). This finding contributes to the fact that the type of secondary metabolite extraction method of rambutan leaf endophytic bacteria influences the sensivity as an antibacterial.
Downloads
References
Abdallah, R. A. Ben, Jabnoun-Khiareddine, H., & Daami-Remadi, M. (2019). Exploring the beneficial endophytic microorganisms for plant growth promotion and crop protection: Elucidation of some bioactive secondary metabolites involved in both effects. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms: Discovery and Applications. https://doi.org/10.1007/978-981-13-5862-3_16
Afzaal, M., Saeed, F., Bibi, M., Ejaz, A., Shah, Y. A., Faisal, Z., Ateeq, H., Akram, N., Asghar, A., & Shah, M. A. (2023). Nutritional, pharmaceutical, and functional aspects of rambutan in industrial perspective: An updated review. Food Science and Nutrition, February, 1–11. https://doi.org/10.1002/fsn3.3379
Ahmad, I., & Chua, P. C. (2013). Trends in production and trade of tropical fruits in ASEAN countries. Acta Horticulturae, 975, 559–580. https://doi.org/10.17660/ActaHortic.2013.975.73
Ali, U., Karim, K. J. B. A., & Buang, N. A. (2015). A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polymer Reviews, 55(4), 678–705. https://doi.org/10.1080/15583724.2015.1031377
Alibi, S., Crespo, D., & Navas, J. (2021). Plant-derivatives small molecules with antibacterial activity. Antibiotics, 10(3), 1–19. https://doi.org/10.3390/antibiotics10030231
Amaha, N. D., Mebrahtu, S. G., & Abdu, N. (2022). Saponins and their synergistic antibacterial activity with traditional antibiotics against Staphylococcus aureus and Escherichia coli: Review. Qeios, November, 1–14. https://doi.org/10.32388/YO91ZE
Andalib, R., Majid, M. Z. A., Hussin, M. W., Ponraj, M., Keyvanfar, A., Mirza, J., & Lee, H. S. (2016). Optimum concentration of Bacillus megaterium for strengthening structural concrete. Construction and Building Materials, 118, 180–193. https://doi.org/10.1016/j.conbuildmat.2016.04.142
Anjum, N., & Chandra, R. (2015). Endophytic bacteria: Optimizaton of isolation procedure from various medicinal plants and their preliminary characterization. Asian Journal of Pharmaceutical and Clinical Research, 8(4), 233–238.
Arnold, A. Elizabeth, & Lutzoni, F. (2007). Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology, 88(3), 541–549. https://doi.org/10.1890/05-1459
Arnold, A E, Mejia, L. C., Rojas, E. I., Maynard, Z., Robbins, N., & Herre, E. A. (2003). Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences of the United States of America, 100(26), 15649–15654.
Babu, A. K., Kumaresan, G., Raj, V. A. A., & Velraj, R. (2018). Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renewable and Sustainable Energy Reviews, 536–556. https://doi.org/10.1016/j.rser.2018.04.002
Beiranvand, M., Amin, M., Hashemi-Shahraki, A., Romani, B., Yaghoubi, S., & Sadeghi, P. (2017). Antimicrobial activity of endophytic bacterial populations isolated from medical plants of Iran. Iranian Journal of Microbiology, 9(1), 11–18.
Bogas, A. C., Ferreira, A. J., Araújo, W. L., Astolfi-Filho, S., Kitajima, E. W., Lacava, P. T., & Azevedo, J. L. (2015). Endophytic bacterial diversity in the phyllosphere of Amazon Paullinia cupana associated with asymptomatic and symptomatic anthracnose. SpringerPlus, 4(1). https://doi.org/10.1186/s40064-015-1037-0
Breil, C., Vian, M. A., Zemb, T., Kunz, W., & Chemat, F. (2017). “Bligh and Dyer” and Folch methods for solid–liquid–liquid extraction of lipids from microorganisms. Comprehension of solvatation mechanisms and towards substitution with alternative solvents. International Journal of Molecular Sciences, 18(4), 1–21. https://doi.org/10.3390/ijms18040708
Cacique, A. P., Barbosa, É. S., de Pinho, G. P., & Silvério, F. O. (2020). Maceration extraction conditions for determining the phenolic compounds and the antioxidant activity of catharanthus roseus (L.) g. don. Ciencia e Agrotecnologia, 44, 1–12. https://doi.org/10.1590/1413-7054202044017420
Carvalho, R. S., Carollo, C. A., de Magalhães, J. C., Palumbo, J. M. C., Boaretto, A. G., Nunes e Sá, I. C., Ferraz, A. C., Lima, W. G., de Siqueira, J. M., & Ferreira, J. M. S. (2018). Antibacterial and antifungal activities of phenolic compound-enriched ethyl acetate fraction from Cochlospermum regium (mart. Et. Schr.) Pilger roots: Mechanisms of action and synergism with tannin and gallic acid. South African Journal of Botany, 114, 181–187. https://doi.org/10.1016/j.sajb.2017.11.010
Chai, K. F., Chang, L. S., Adzahan, N. M., Karim, R., Rukayadi, Y., & Ghazali, H. M. (2019). Physicochemical properties and toxicity of cocoa powder-like product from roasted seeds of fermented rambutan (Nephelium lappaceum L.) fruit. Food Chemistry, 271, 298–308. https://doi.org/10.1016/j.foodchem.2018.07.155
Chigurupati, S., Vijayabalan, S., Karunanidhi, A., Krishnan Selvarajan, K., Nanda, S. S., & Satpathy, R. (2019). Antidiabetic, antioxidant and in silico studies of bacterial endosymbiont inhabiting Nephelium lappaceum L. . Ovidius University Annals of Chemistry, 30(2), 95–100. https://doi.org/10.2478/auoc-2019-0017
Chigurupati, S., Vijayabalan, S., Selvarajan, K. K., Hashish, N. E., Mani, V., Ahmed, E. S., & Das, S. (2019). Identification of Nephelium lappaceum leaves phenolic and flavonoid component with radical scavenging, antidiabetic and antibacterial potential. Indian Journal of Traditional Knowledge, 18(2), 360–365.
Cushnie, T. P. T., & Lamb, A. J. (2011). Recent advances in understanding the antibacterial properties of flavonoids. International Journal of Antimicrobial Agents, 38(2), 99–107. https://doi.org/10.1016/j.ijantimicag.2011.02.014
Dai, J. X., Liu, X. M., & Wang, Y. J. (2014). Diversity of endophytic bacteria in Caragana microphylla grown in the desert grassland of the Ningxia Hui Autonomous Region of China. Genetics and Molecular Research, 13(2), 2349–2358. https://doi.org/10.4238/2014.April.3.7
de Santana Santos, A., de Souza Oliveira, A. K., Pereira, R. O., Junior, E. V. B., de Lima Sayão, A., & de Oliveira e Silva, A. M. (2021). Composition and Biological Properties of Rambutan (Nephelium lappaceum). Phytopharmaceuticals: Potential Therapeutic Applications, 403–436. https://doi.org/10.1002/9781119682059.ch21
Dewi, P., Putri, A. R., Bintari, S. H., & Mubarok, I. (2022). Uji Efektivitas Ekstrak Buah Ketapang (Terminalia catappa) terhadap Bakteri Escherichia coli dan Bacillus subtilis. Life Science, 8(1), 18–24. http://journal.unnes.ac.id/sju/index.php/LifeSci
Dinos, G. P., Athanassopoulos, C. M., Missiri, D. A., Giannopoulou, P. C., Vlachogiannis, I. A., Papadopoulos, G. E., Papaioannou, D., & Kalpaxis, D. L. (2016). Chloramphenicol derivatives as antibacterial and anticancer agents: Historic problems and current solutions. Antibiotics, 5(2). https://doi.org/10.3390/antibiotics5020020
Dong, S., Yang, X., Zhao, L., Zhang, F., Hou, Z., & Xue, P. (2020). Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against foodborne pathogenic bacteria. Industrial Crops and Products, 149(August 2019), 112350. https://doi.org/10.1016/j.indcrop.2020.112350
Ergüden, B. (2021). Phenol group of terpenoids is crucial for antibacterial activity upon ion leakage. Letters in Applied Microbiology, 73(4), 438–445. https://doi.org/10.1111/lam.13529
Fajeriyati, N., & Andika. (2017). Uji Aktivitas Antibakteri Ekstrak Etanol Rimpang Kencur (Kaempferia galanga L) pada Bakteri Bacillus subtilis dan Escherichia coli. Journal of Current Pharmaceutical Sciences., 1(1), 36–41.
Fan, Y., Gao, L., Chang, P., & Li, Z. (2020). Endophytic fungal community in grape is correlated to foliar age and domestication. Annals of Microbiology, 70(1), 4–11. https://doi.org/10.1186/s13213-020-01574-9
Farha, A. K., Yang, Q. Q., Kim, G., Li, H. Bin, Zhu, F., Liu, H. Y., Gan, R. Y., & Corke, H. (2020). Tannins as an alternative to antibiotics. Food Bioscience. (38) 100751. https://doi.org/10.1016/j.fbio.2020.100751
Ghosh, K., Ray, A. K., & Sen, S. K. (2002). Characterization of bacilli isolated from the gut of rohu, labeo rohita, fingerlings and its significance in digestion. Journal of Applied Aquaculture, 12(3), 33–42. https://doi.org/10.1300/J028v12n03_04
Giannopoulou, P. C., Missiri, D. A., Kournoutou, G. G., Sazakli, E., Papadopoulos, G. E., Papaioannou, D., Dinos, G. P., Athanassopoulos, C. M., & Kalpaxis, D. L. (2019). New chloramphenicol derivatives from the viewpoint of anticancer and antimicrobial activity. Antibiotics, 8(1), 1–16. https://doi.org/10.3390/antibiotics8010009
González, V., & Tello, M. L. (2011). The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Diversity, 47, 29–42. https://doi.org/10.1007/s13225-010-0073-x
Gouda, S., Das, G., Sen, S. K., Shin, H. S., & Patra, J. K. (2016). Endophytes: A treasure house of bioactive compounds of medicinal importance. Frontiers in Microbiology, 7(SEP), 1–8. https://doi.org/10.3389/fmicb.2016.01538
Griffiths, M. W., & Schraft, H. (2017). Bacillus cereus Food Poisoning. In Foodborne Diseases: Third Edition (Third Edit). Elsevier Inc. https://doi.org/10.1016/B978-0-12-385007-2.00020-6
Hammado, N., & Illing, I. (2013). Identifikasi Senyawa Bahan Aktif Alkaloid Pada Tanaman Lahuna (Eupatorium odoratum). Jurnal Dinamika, 04(2), 1–18.
Horton, M. W., Bodenhausen, N., Beilsmith, K., Meng, D., Muegge, B. D., Subramanian, S., Vetter, M. M., Vilhjálmsson, B. J., Nordborg, M., Gordon, J. I., & Bergelson, J. (2014). Genome-wide association study of Arabidopsis thaliana leaf microbial community. Nature Communications, 5(May), 1–7. https://doi.org/10.1038/ncomms6320
Iguchi, A., Iyoda, S., Kikuchi, T., Ogura, Y., Katsura, K., Ohnishi, M., Hayashi, T., & Thomson, N. R. (2015). A complete view of the genetic diversity of the Escherichia coli O-antigen biosynthesis gene cluster. DNA Research, 22(1), 101–107. https://doi.org/10.1093/dnares/dsu043
Irma, A., Meryandini, A., & Rupaedah, B. (2018). Biofungicide producing bacteria: An in vitro inhibitor of Ganoderma boninense. HAYATI Journal of Biosciences, 25(4), 151–159. https://doi.org/10.4308/hjb.25.4.151
Islam, N., Choi, J., & Baek, K. H. (2018). Antibacterial Activities of Endophytic Bacteria Isolated from Taxus brevifolia Against Foodborne Pathogenic Bacteria. Foodborne Pathogens and Disease, 15(5), 269–276. https://doi.org/10.1089/fpd.2017.2357
Jahurul, M. H. A., Azzatul, F. S., Sharifudin, M. S., Norliza, M. J., Hasmadi, M., Lee, J. S., Patricia, M., Jinap, S., Ramlah George, M. R., Firoz Khan, M., & Zaidul, I. S. M. (2020). Functional and nutritional properties of rambutan (Nephelium lappaceum L.) seed and its industrial application: A review. Trends in Food Science and Technology, (99). 367–374. https://doi.org/10.1016/j.tifs.2020.03.016
Joseph, M. R. P., Al-Hakami, A. M., Assiry, M. M., Jamil, A. S., Assiry, A. M., Shaker, M. A., & Hamid, M. E. (2015). In vitro anti-yeast activity of chloramphenicol: A preliminary report. Journal de Mycologie Medicale, 25(1), 17–22. https://doi.org/10.1016/j.mycmed.2014.10.019
Kogel, K. H., Franken, P., & Hückelhoven, R. (2006). Endophyte or parasite - what decides? Current Opinion in Plant Biology, 9(4), 358–363. https://doi.org/10.1016/j.pbi.2006.05.001
Kolton, M., Graber, E. R., Tsehansky, L., Elad, Y., & Cytryn, E. (2017). Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytologist, 213(3), 1393–1404. https://doi.org/10.1111/nph.14253
Krishnamoorthy, G., Wolloscheck, D., Weeks, J. W., Croft, C., Rybenkov, V. V., & Zgurskaya, H. I. (2016). Breaking the permeability barrier of Escherichia coli by controlled Hyperporination of the outer membrane. Antimicrobial Agents and Chemotherapy, 60(12), 7372–7381. https://doi.org/10.1128/AAC.01882-16
Kusbandari, A., & Susanti, H. (2017). Determination of total phenolic content and antioxidant activitity of methanol extract of Maranta arundinacea L fresh leaf and tuber. IOP Conference Series: Materials Science and Engineering, 259(1). https://doi.org/10.1088/1757-899X/259/1/012010
Lin, X., Tfaily, M. M., Steinweg, J. M., Chanton, P., Esson, K., Yang, Z. K., Chanton, J. P., Cooper, W., Schadt, C. W., & Kostka, J. E. (2014). Microbial community stratification linked to utilization of carbohydrates and phosphorus limitation in a Boreal Peatland at Marcell Experimental Forest, Minnesota, USA. Applied and Environmental Microbiology, 80(11), 3518–3530. https://doi.org/10.1128/AEM.00205-14
Lisdiana, & Dewi, F. K. (2017). Effects of Rambutan Peel Extract to The Number of Erythrocytes and Haemoglobin in Rats Exposed to Cigarette Smoke. Journal of Physics: Conference Series, 755(1), 2–8. https://doi.org/10.1088/1742-6596/755/1/011001
Masegi, J. M. G., Puspawati, G. A. K. D., & Wiadnyani, A. A. I. S. (2020). Pengaruh Jenis Pelarut terhadap Aktivitas Antioksidan Ekstrak Meniran (Phyllanthus niruri L.). Jurnal Ilmu Dan Teknologi Pangan (ITEPA), 9(4), 458. https://doi.org/10.24843/itepa.2020.v09.i04.p10
Maulidia, V., Soesanto, L., Syamsuddin, Khairan, K., Hamaguchi, T., Hasegawa, K., & Sriwati, R. (2020). Secondary metabolites produced by endophytic bacteria against the root-knot nematode (Meloidogyne sp.). Biodiversitas, 21(11), 5270–5275. https://doi.org/10.13057/biodiv/d211130
Mohamad, O. A. A., Li, L., Ma, J. B., Hatab, S., Xu, L., Guo, J. W., Rasulov, B. A., Liu, Y. H., Hedlund, B. P., & Li, W. J. (2018). Evaluation of the antimicrobial activity of endophytic bacterial populations from Chinese traditional medicinal plant licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus Against Verticillium dahliae. Frontiers in Microbiology, 9(MAY), 1–14. https://doi.org/10.3389/fmicb.2018.00924
Naviglio, D., Scarano, P., Ciaravolo, M., & Gallo, M. (2019). Rapid solid-liquid dynamic extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods, 8(7), 1–22. https://doi.org/10.3390/foods8070245
Nikaido, H. (2003). Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiology and Molecular Biology Reviews, 67(4), 593–656. https://doi.org/10.1128/mmbr.67.4.593-656.2003
Nikaido, H., & Pagès, J. M. (2012). Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiology Reviews, 36(2), 340–363. https://doi.org/10.1111/j.1574-6976.2011.00290.x
Nofiani, R., Nurbetty, S., & Sapar, A. (2009). AKTIVITAS ANTIMIKROBA EKSTRAK METANOL BAKTERI BERASOSIASI SPONS DARI PULAU LEMUKUTAN, KALIMANTAN BARAT. 1(2), 33–41.
Oono, R., Lefevre, E., Simha, A., & Lutzoni, F. (2015). A comparison of the community diversity of foliar fungal endophytes between seedling and adult loblolly pines (Pinus taeda). Fungal Biology, 119(10), 917–928.
Pancher, M., Ceol, M., Corneo, P. E., Longa, C. M. O., Yousaf, S., Pertot, I., & Campisano, A. (2012). Fungal endophytic communities in grapevines (Vitis vinifera L.) Respond to crop management. Applied and Environmental Microbiology, 78(12), 4308–4317. https://doi.org/10.1128/AEM.07655-11
Pandiangan, F. S., Biologi, D., Matematika, F., Ilmu, D. A. N., Alam, P., & Utara, U. S. (2014). Isolasi dan Uji Ekstrak Metanol Bakteri Endofit Tapak Dara (Catharanthus Roseus) dalam Menghambat Pertumbuhan Beberapa Mikroba Patogen [Doctoral dissertation, Universitas Sumatera Utara].
Patil, R. H., Patil, M. P., & Maheshwari, V. L. (2016). Bioactive Secondary Metabolites From Endophytic Fungi: A Review of Biotechnological Production and Their Potential Applications. Studies in Natural Products Chemistry, 49(December), 189–205. https://doi.org/10.1016/B978-0-444-63601-0.00005-3
Pratiwi, B. E. (2015). Isolasi dan Skrining Fitokimia Bakteri Endofit dari Daun Rambutan (Nephelium lappaceum L.) yang Berpotensi Sebagai Antibakteri. [Thesis, UIN Syarif Hidayatullah Jakarta].Campus Repository. https://repository.uinjkt.ac.id/dspace/bitstream/123456789/36992/1/Brasti%20Eka%20Pratiwi-FKIK.pdf
Puspita, F., Ali, M., & Pratama, R. (2017). Isolasi dan Karakterisasi Morfologi dan Fisiologi Bakteri Bacillus sp. Endofitik dari Tanaman Kelapa Sawit (Elaeis guineensis Jacq.) Isolation and Characterization of Morphology and Physiology of Endophytic Bacillus sp. from Oil Palm Plants (Elaeis guinee. J. Agrotek. Trop, 6(2), 44–49.
Putri, M. F., Fifendy, M., & Putri, D. H. (2018). Diversitas Bakteri Endofit Pada Daun Muda Dan Tua Tumbuhan Muda. Eksakta, 19(1).
Putri, R., Supriyanta, J., & Adhil, D. A. (2021). Formulasi dan Uji Aktivitas Sediaan Masker Gel Peel Off Ekstrak Etanol 70% Daun Rambutan (Nephelium Lappaceum L.) Terhadap Propionibacterium Acnes. Journal of Pharmaceutical and Health Research, 2(1), 12–20. https://doi.org/10.47065/jharma.v2i1.836
Rahman, L., Mukhtar, A., Ahmad, S., Rahman, L., Ali, M., Saeed, M., & Shinwari, Z. K. (2022). Endophytic bacteria of Fagonia indica Burm. F revealed to harbour rich secondary antibacterial metabolites. PLoS ONE, 17(12 December), 1–18. https://doi.org/10.1371/journal.pone.0277825
Rahman, L., Shinwari, Z. K., Iqrar, I., Rahman, L., & Tanveer, F. (2017). An assessment on the role of endophytic microbes in the therapeutic potential of Fagonia indica. Annals of Clinical Microbiology and Antimicrobials, 16(1), 1–12. https://doi.org/10.1186/s12941-017-0228-7
Rattanata, N., Klaynongsruang, S., Leelayuwat, C., Limpaiboon, T., Lulitanond, A., Boonsiri, P., Chio-Srichan, S., Soontaranon, S., Rugmai, S., & Daduang, J. (2016). Gallic acid conjugated with gold nanoparticles: Antibacterial activity and mechanism of action on foodborne pathogens. International Journal of Nanomedicine, 11, 3347–3356. https://doi.org/10.2147/IJN.S109795
Sabu, R., & Radhakrishnan, E. K. (2016). Bioprospecting of Endophytic Bacteria from Zingiber officinale with Antibacterial Activities. International Journal of Current Microbiology and Applied Sciences, 5(9), 462–467. https://doi.org/10.20546/ijcmas.2016.509.050
Sadeghi, F., Samsampour, D., Seyahooei, M. A., Bagheri, A., & Soltani, J. (2019). Diversity and Spatiotemporal Distribution of Fungal Endophytes Associated with Citrus reticulata cv. Siyahoo. Current Microbiology, 76(3), 279–289. https://doi.org/10.1007/s00284-019-01632-9
Sadikin, N. A. N., Bintari, S. H., Widiatningrum, T., & Dewi, P. (2021). Isolasi, Karakterisasi, dan Uji Aktivitas Antibakteri dari Bakteri Endofit Daun Kelor (Moringa oleifera). Life Science, 10(2), 109–119. https://doi.org/10.15294/lifesci.v10i2.54441
Sari, S. A., Pujiyanto, S., & Suprihadi, A. (2020). Antibacterial activity tests of isolate endophytic bacteria from the tea plant (Camellia sinensis) againts Staphylococcus aureus and Staphylococcus epidermidis. Journal of Physics: Conference Series, 1524(1). https://doi.org/10.1088/1742-6596/1524/1/012067
Sarjono, P. R., Putri, L. D., Budiarti, C. E., Mulyani, N. S., Ngadiwiyana, Ismiyarto, Kusrini, D., & Adiwibawa Prasetya, N. B. (2019). Antioxidant and antibacterial activities of secondary metabolite endophytic bacteria from papaya leaf (Carica papaya L.). IOP Conference Series: Materials Science and Engineering, 509(1). https://doi.org/10.1088/1757-899X/509/1/012112
Sharma, M., & Mallubhotla, S. (2022). Diversity, Antimicrobial Activity, and Antibiotic Susceptibility Pattern of Endophytic Bacteria Sourced From Cordia dichotoma L. Frontiers in Microbiology, 13(May), 1–17. https://doi.org/10.3389/fmicb.2022.879386
Shinde, A. B., & Mulay, Y. (2015). Phytochemical Analysis and Antibacterial Properties of Some Selected Indian Medicinal Plants. International Journal of Current Microbiology and Applied Sciences, 4(2), 228-235.
Sipriyadi, Masrukhin, Wibowo, R. H., Darwis, W., Yudha, S., Purnaningsih, I., & Siboro, R. (2022). Potential Antimicrobe Producer of Endophytic Bacteria from Yellow Root Plant (Arcangelisia flava (L.)) Originated from Enggano Island. International Journal of Microbiology, 2022. https://doi.org/10.1155/2022/6435202
Soliha, A. M., Heliawati, L., & Warnasih, S. (2021). Identification of Antibacterial Compounds From Endophytic Bacterial Extract of Green Grass Cincau Plant (Premna Oblongifolia Merr). Helium: Journal of Science and Applied Chemistry, 1(2), 46–50. https://doi.org/10.33751/helium.v1i2.4538
Suhandono, S., Kusumawardhani, M. K., & Aditiawati, P. (2016). Isolation and Molecular Identification of Endophytic Bacteria From Rambutan Fruits (Nephelium lappaceum L.) Cultivar Binjai. HAYATI Journal of Biosciences, 23(1), 39–44. https://doi.org/10.1016/j.hjb.2016.01.005
Sulaiha, Mustikaningtyas, Widiatningrum, & Dewi. (2022). Senyawa Bioaktif Trichoderma erinaceum dan Trichoderma koningiopsis Serta Potensinya Sebagai Antibakteri. Life Science, 11(2), 120–131.
Sulistiyani, S., Ardyati, T., & Winarsih, S. (2016). Antimicrobial and Antioxidant Activity of Endophyte Bacteria Associated with Curcuma longa Rhizome. The Journal of Experimental Life Sciences, 6(1), 45–51. https://doi.org/10.21776/ub.jels.2016.006.01.11
Sulistiyaningsih, S., S, N., Wicaksono, I., & Budiman, A. (2017). Antibacterial activity of ethanol extract and fraction of Rambutan leaf (Nephelium lappaceum) against Pseudomonas aeruginosa multiresistant. National Journal of Physiology, Pharmacy and Pharmacology, 7(11), 1. https://doi.org/10.5455/njppp.2017.7.0935926102017
Suryani, N. C., Permana, D. G. M., & Jambe, A. A. G. N. A. (2015). Pengaruh Jenis Pelarut Terhadap Kandungan Total Flavonoid dan Aktivitas Antioksidan Ekstrak Daun Matoa (Pometia pinnata). Journal of the Japanese Society of Pediatric Surgeons, 18(2), 33–37. https://ojs.unud.ac.id/index.php/itepa/article/view/22645
Tallei, T. E., Linelejan, Y. T., Umboh, S. D., Adam, A. A., Muslem, & Idroes, R. (2020). Endophytic Bacteria isolated from the leaf of Langusei (Ficus minahassae Tesym. & de Vr.) and their antibacterial activities. IOP Conference Series: Materials Science and Engineering, 796(1), 5–12. https://doi.org/10.1088/1757-899X/796/1/012047
Tambun, R., Alexander, V., & Ginting, Y. (2021). Performance comparison of maceration method, soxhletation method, and microwave-assisted extraction in extracting active compounds from soursop leaves (Annona muricata): A review. IOP Conference Series: Materials Science and Engineering, 1122(1), 012095. https://doi.org/10.1088/1757-899x/1122/1/012095
Utami, L. A., & Putri, D. H. (2020). The Effect of Ethanol Solvent Concentration on Antimicrobial Activities The Extract of Andalas Endophytic Bacteria (Morus Macroura Miq.) Fermentation Product. Eksakta : Berkala Ilmiah Bidang MIPA, 21(1), 1–6. https://doi.org/10.24036/eksakta/vol21-iss1/210
Vendan, R. T., Yu, Y. J., Lee, S. H., & Rhee, Y. H. (2010). Diversity of endophytic bacteria in ginseng and their potential for plant growth promotion. Journal of Microbiology, 48(5), 559–565. https://doi.org/10.1007/s12275-010-0082-1
Verdiana, M., Widarta, I. W. R., & Permana, I. D. G. M. (2018). Pengaruh Jenis Pelarut pada Ekstraksi Menggunakan Gelombang Ultrasonik Terhadap Aktivitas Antioksidan Ekstrak Kulit Buah Lemon (Citrus limon (Linn.) Burm F.). Jurnal Ilmu Dan Teknologi Pangan (ITEPA), 7(4), 213. https://doi.org/10.24843/itepa.2018.v07.i04.p08
Verma, S. K., Kharwar, R. N., Gond, S. K., Kingsley, K., & White, J. F. (2019). Exploring Endophytic Communities of Plants: Methods for Assessing Diversity, Effects on Host Development and Potential Biotechnological Applications. In Seed Endophytes: Biology and Biotechnology (Issue April). https://doi.org/10.1007/978-3-030-10504-4
Vijayalakshmi, R., Kairunnisa, K., Sivvaswamy, N., Dharan, S. S., & Natarajan, S. (2016). Enzyme production and antimicrobial activity of endophytic bacteria isolated from medicinal plants. Indian Journal of Science and Technology, 9(14). https://doi.org/10.17485/ijst/2016/v9i14/83143
Walitang, D. I., Kim, C. G., Kim, K., Kang, Y., Kim, Y. K., & Sa, T. (2018). The influence of host genotype and salt stress on the seed endophytic community of salt-sensitive and salt-tolerant rice cultivars. BMC Plant Biology, 18(1), 1–16. https://doi.org/10.1186/s12870-018-1261-1
Wang, M., Li, Z., Zhang, Y., Li, Y., Li, N., Huang, D., & Xu, B. (2021). Interaction with teichoic acids contributes to highly effective antibacterial activity of graphene oxide on Gram-positive bacteria. Journal of Hazardous Materials, 412(November 2020). https://doi.org/10.1016/j.jhazmat.2021.125333
Wu, W., Chen, W., Liu, S., Wu, J., Zhu, Y., Qin, L., & Zhu, B. (2021). Beneficial Relationships Between Endophytic Bacteria and Medicinal Plants. Frontiers in Plant Science, 12(April), 1–13. https://doi.org/10.3389/fpls.2021.646146
Xie, Y., Yang, W., Tang, F., Chen, X., & Ren, L. (2015). Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Current Medicinal Chemistry, 22(1), 132–149. https://doi.org/10.2174/0929867321666140916113443
Yan, Y., Li, X., Zhang, C., Lv, L., Gao, B., & Li, M. (2021). Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics, 10(3). https://doi.org/10.3390/antibiotics10030318
Zgurskaya, H. I., López, C. A., & Gnanakaran, S. (2015). Permeability Barrier of Gram-Negative Cell Envelopes and Approaches to Bypass It. ACS Infectious Diseases, 1(11), 512–522. https://doi.org/10.1021/acsinfecdis.5b00097